Publications co-authored with David Rogers
| 16. | Chaudhuri, S.; Huynh, B.C.; Amory, R.; Rogers, D.M.; Cranney, A.; Martínez-Martínez, L.A.; Macrì, T.; Jones, G.; Sheridan, E.; Khedkar, A.; Mineh, L.; Inzani, K.; Hirst J.D., Challenges and Advances in the Simulation of Targeted Covalent Inhibitors Using Quantum Computing. J. Phys. Chem. Lett., 16, 8536−8545 (2025). DOI: http://dx.doi.org/10.1021/acs.jpclett.5c01680  |
| 15. | Chaudhuri, S.; Rogers, D.M.; Hayes, C.J.; Inzani, K.; Hirst, J.D., Quantum Chemical Characterization of Rotamerism in Thio-Michael Additions for Targeted Covalent Inhibitors. J. Chem. Inf. Mod., 64, 7687–7697 (2024). DOI: http://dx.doi.org/10.1021/acs.jcim.4c01379  |
| 14. | Rogers, D.M.; Do, H.; Hirst, J.D., An Improved Diabatization Scheme for Computing the Electronic Circular Dichroism of Proteins. J. Phys. Chem. B, 128, 7350–7361 (2024). DOI: http://dx.doi.org/10.1021/acs.jpcb.4c02582 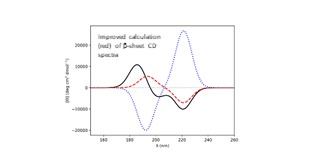 |
| 13. | Douaki, A.; Stuber, A.; Hengsteler, J.; Momotenko, D.; Rogers, D.M.; Hirst, J.D.; Nakatsuka, N.; Garoli, D., Theoretical visualization of divalent cations effects on aptamer recognition of neurotransmitter targets. Chem. Commun., 59, 14713–14716 (2023). DOI: http://dx.doi.org/10.1039/d3cc04334g  |
| 12. | Griffiths, R.C.; Smith, F.R.; Li, D.; Wyatt, J.; Rogers, D.M.; Long, J.E.; Cusin, L.M.L.; Tighe, P.J.; Layfield, R.; Hirst, J.D. & Mitchell, N.J. , Cysteine-Selective Modification of Peptides and Proteins via Desulfurative C-C Bond Formation. Chem. Eur. J., 29, e202202503 (2023). DOI: http://dx.doi.org/10.1002/chem.202202503  |
| 11. | Rogers, D.M.; Do, H. & Hirst, J.D., Electronic Circular Dichroism of Proteins Computed Using a Diabatisation Scheme. Mol. Phys., 121, e2133748 (2023). DOI: http://dx.doi.org/10.1080/00268976.2022.2133748  |
| 10. | Silva, A.F.; Guest, E.E.; Falcone, B.N.; Pickett, S.D.; Rogers, D.M.; Hirst, J.D., Free energy perturbation calculations of tetrahydroquinolines complexed to the first bromodomain of BRD4. Mol. Phys., 121, e2124201 (2023). DOI: http://dx.doi.org/10.1080/00268976.2022.2124201  |
| 9. | Segatta, F., Rogers, D.M., Dyer, N.T., Guest, E.E., Li, Z., Do, H., Nenov, A., Garavelli, M., Hirst, J.D., Near-ultraviolet circular dichroism and two-dimensional spectroscopy of polypeptides. Molecules, 26, 396 (2021). DOI: http://dx.doi.org/10.3390/molecules26020396  |
| 8. | Jiang, L., Rogers, D.M., Hirst, J.D. & Do, H., Force fields for macromolecular assemblies containing diketopyrrolopyrrole and thiophene. J. Chem. Theor. Comput., 16, 5150–5162 (2020). DOI: http://dx.doi.org/10.1021/acs.jctc.0c00399  |
| 7. | Hanson-Heine, M.W.D., Rogers, D.M., Woodward, S. & Hirst, J.D. , Dewar benzene ground states found in cyclacene nanobelts. J. Phys. Chem. Lett., 11, 3769–3772 (2020). DOI: http://dx.doi.org/10.1021/acs.jpclett.0c01027 |
| 6. | Rogers, D.M, Jasim, S.B, Dyer, N.T, Auvray, F, Rèfrègiers, M, Hirst, J.D., Electronic circular dichroism of proteins. Chem, 5, 2751–2774 (2019). DOI: http://dx.doi.org/10.1016/j.chempr.2019.07.008 |
| 5. | Rogers, D.M., Hirst, J.D., Lee, E.P.F. & Wright, T.G., Ab Initio Study of the Toluene Dimer. Chem. Phys. Lett., 427, 410–413 (2006). DOI: http://dx.doi.org/10.1016/j.cplett.2006.07.022  |
| 4. | Rogers, D.M., Besley, N.A., O'Shea, P. & Hirst, J.D., Modeling the Absorption Spectrum of Tryptophan in Proteins. J. Phys. Chem. B, 109, 23061–23069 (2005). DOI: http://dx.doi.org/10.1021/jp053309j |
| 3. | Rogers, D.M. & Hirst, J.D., First Principles Calculations of Protein Circular Dichroism in the Near-Ultraviolet. Biochemistry, 43, 11092–11102 (2004). DOI: http://dx.doi.org/10.1021/bi049031n  |
| 2. | Rogers, D.M. & Hirst, J.D., Calculations of Protein Circular Dichroism from First Principles. Chirality, 16, 234–243 (2004). DOI: http://dx.doi.org/10.1002/chir.20018 |
| 1. | Rogers, D.M. & Hirst, J.D., Ab Initio Studies of Aromatic Side-Chains in Gas Phase and Solution. J. Phys. Chem. A, 107, 11191–11200 (2003). DOI: http://dx.doi.org/10.1021/jp036081d |


